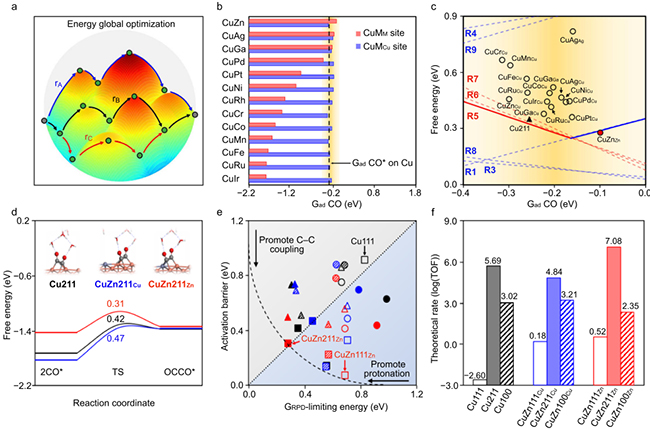
Electrochemical CO2 reduction (CO2R) to ethylene and ethanol enables the long-term storage of renewable electricity in valuable multi-carbon (C2+) chemicals. However, carbon–carbon (C–C) coupling, the rate-determining step in CO2R to C2+ conversion, has low efficiency and poor stability, especially in acid conditions. Here we find that, through alloying strategies, neighbouring binary sites enable asymmetric CO binding energies to promote CO2-to-C2+ electroreduction beyond the scaling-relation-determined activity limits on single-metal surfaces. We fabricate experimentally a series of Zn incorporated Cu catalysts that show increased asymmetric CO* binding and surface CO* coverage for fast C–C coupling and the consequent hydrogenation under electrochemical reduction conditions. Further optimization of the reaction environment at nanointerfaces suppresses hydrogen evolution and improves CO2 utilization under acidic conditions. We achieve, as a result, a high 31 ± 2% single-pass CO2-to-C2+ yield in a mild-acid pH 4 electrolyte with >80% single-pass CO2 utilization efficiency. In a single CO2R flow cell electrolyzer, we realize a combined performance of 91 ± 2% C2+ Faradaic efficiency with notable 73 ± 2% ethylene Faradaic efficiency, 31 ± 2% full-cell C2+ energy efficiency, and 24 ± 1% single-pass CO2 conversion at a commercially relevant current density of 150 mA cm−2 over 150 h.