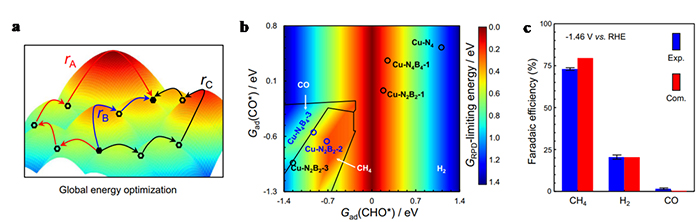
Electrochemical CO2 conversion to methane, powered by intermittent renewable electricity, provides an entrancing opportunity to both store renewable electric energy and utilize emitted CO2. Copper-based single atom catalysts are promising candidates to restrain C-C coupling, suggesting feasibility in further protonation of CO* to CHO* for methane production. In theoretical studies herein, we find that introducing boron atoms into the first coordination layer of Cu-N4 motif facilitates the binding of CO* and CHO* intermediates, which favors the generation of methane. Accordingly, we employ a co-doping strategy to fabricate B-doped Cu-Nx atomic configuration (Cu-NxBy), where Cu-N2B2 is resolved to be the dominant site. Compared with Cu-N4 motifs, as-synthesized B-doped Cu-Nx structure exhibits a superior performance towards methane production, showing a peak methane Faradaic efficiency of 73% at −1.46 V vs. RHE and a maximum methane partial current density of −462 mA cm−2 at −1.94 V vs. RHE. Extensional calculations utilizing two-dimensional reaction phase diagram analysis together with barrier calculation help to gain more insights into the reaction mechanism of Cu-N2B2 coordination structure.